Imports
Biological Imports
Certain biological materials are regulated by federal agencies for international importation and interstate transfer. Permits may be required from these agencies. Exempt materials must meet specific criteria and be accompanied by shipping documentation for US Customs and Border Patrol review to avoid confiscation or delays.
The Office of Sponsored Programs requests ancillary review of Material Transfer Agreements of biological materials, which includes animals, viruses and vectors, cell lines, plasmids, and bacterial agents.
If an MTA is not necessary, import review is still required to determine necessary permitting requirements. Email researchsecurity@pitt.edu before shipment.
Import Review will determine the permits required and guide the investigator to the appropriate permitting tools. As the permits are done through online systems, the investigator must apply for the permits themselves.
Please note that all permits MUST be under the receiving investigators name and should match the MTA, if applicable.
Hand carried items via passenger travel DO require appropriate permits and also notification to Customs and Border Protection for inspection. This applies to materials of declared “zero value” such as research materials. CBP does not recommend hand carry if other shipping methods are available.
https://www.cbp.gov/trade/basic-import-export/importing-biological-materials-united-states
See: section on Importing Biological Materials by Passenger Travel
The following is not an exhaustive list of materials that may require documentation or permits. If you have any questions please contact researchsecurity@pitt.edu .
If you are exporting biological materials to another country, export regulations may apply. https://www.tradecompliance.pitt.edu/procedures/shipments
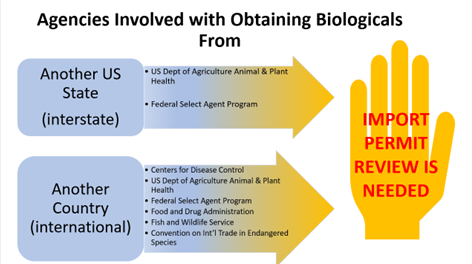
Common Permitting Agencies
Centers for Disease Control (CDC)
International Movement
Oversight: Materials that are or may be infectious to humans
Examples : Human samples (blood, tissues, body fluids)
Vectors of human disease (bats, arthropods, snails)
Human or non-human primate remains
LINK: www.cdc.gov/cpr/ipp/index.htm
United States Department of Agriculture (USDA), Animal and Plant Health Inspection Service (APHIS)
International and Interstate Movement
APHIS Veterinary Services (VS)
Oversight: animals or animal products
Examples: Animals (transgenic animals, tissues, embryos or sperm)
Animal Products (FBS, albumin)
Cell Lines (including Monoclonal antibodies, cell culture supernatants, ascitic fluid, cell extracts, hybridomas)
Recombinant Materials (bacteria, viruses, yeasts/fungi, proteins, hormones, extracts, plasmids, DNA, RNA)
APHIS Organisms and Vectors (OV)
Oversight: Livestock and Poultry Pathogens
Examples: Some avian influenzas, Candida albicans, Enterococcus spp, Klebsiella pneumoniae
Full List here: VS-Regulated Livestock and Poultry Pathogens (Partial List) | Animal and Plant Health Inspection Service (usda.gov)
LINK: Livestock and Poultry Diseases (usda.gov)
APHIS Biotechnology Regulatory Services (BRS)
Oversight: GMO plant pathogens
Examples: any organisms developed using genetic engineering that may pose a risk to plant health
LINK: https://www.aphis.usda.gov/aphis/ourfocus/biotechnology
APHIS Plant Protection and Quarantine (PPQ)
Oversight: Plants and Plant Products
Examples: Plant Pathogens or Pests (plant feeding insects, mites, snails, slugs, and plant pathogenic bacteria, viruses, fungi)
Soil
Seeds
LINK: https://www.aphis.usda.gov/plant-imports/how-to-import
Federal Select Agent Program (FSAP)
International and Interstate Movement
Oversight: possession, use and transfer of biological select agents and toxins, which have the potential to pose a severe threat to public, animal or plant health or to animal or plant products
Examples: Botulinum neurotoxins, Marburg virus, Rift Valley Fever virus
Full list here: https://www.selectagents.gov/sat/list.htm
**Must also have approval from University of Pittsburgh Responsible Official https://www.ehs.pitt.edu/lab-safety/biosafety-program
LINK: https://www.selectagents.gov/sat/index.htm
Food and Drug Administration (FDA)
International Movement
Oversight: all materials covered by FDA in the US are also subject to FDA import review
Examples: Medical products (drugs, vaccines, devices, and biological products)
Radiation emitting devices
Tobacco products
LINK: https://www.fda.gov/industry/import-program-food-and-drug-administration-fda/import-basics
United States Fish and Wildlife Service (FWS)
International and Interstate Movement
Oversight: conserve, protect and enhance fish, wildlife, and plants and their habitats
Examples: wild mammals, bird, reptile, amphibian, fish, mollusk, crustacean, arthropod
LINK: www.fws.gov/le/businesses.html
Convention of International Trade in Endangered Species of Wild Fauna and Flora (CITES)
International Movement
Oversight: International trade in specimens of endangered or threatened wild animals and plants
Examples: Endangered species and their materials (animals and plants)
Full List here: https://cites.org/eng/app/appendices.php
LINK: www.cites.org/eng/disc/how.php
Helpful Office of Trade Compliance Links:
Session 1: Overview of the Importing Process
Session 2: CDC and USDA Permitting Review
Helpful Links:
2021 CDC Import Permit Regulations Webinar
https://www.cdc.gov/orr/ipp/webcast-2021/index.htm
CDC Biological Imports Webinar (32 min)
https://www.youtube.com/watch?v=OUE6HFEzCls
USDA eFILE Permitting Assistant Tutorial (4 min)
https://www.youtube.com/watch?v=r-LkAgRc9pQ
USDA Organisms and Vectors Webinar (14 min)
https://www.youtube.com/watch?v=jBF0ubFAXkg
FDA Import Process (11 min)
https://www.youtube.com/watch?v=PwF-ki-oIDw
